台灣醫療器材登記法規問題集
永輝提供公司註冊、薪酬、會計、稅務、盡職調查、法定審計等服務,服務對象爲臺灣外商企業(WFOE),其母公司來自世界各國。
我們與海外同仁使用雲端系統協同為客戶服務
聯絡人: 朱鍵彰 協理
電話 : +886-2-2717-0515 E103
手機 : +886-939-357-735
電郵:sales.taiwan@evershinecpa.com
HLF-TW-10
請問台灣對於醫療器材的歸類方式為何?它的正式名稱為何?
不同歸類管理強度有何差異?它的政府管轄機構為何?網頁?
What are the classifications of medical devices in Taiwan?
What is its official name? What is the intensity of management of different classifications?
What is the governmental authority of medical devices? Website?
Evershine RD:
判斷是否屬列管之醫療器材可先查詢我國醫療器材分類分級管理辦法之規定,產品若符合任一分類項目即屬醫療器材列管。
若為輸入產品,可參考輸入國家之規定,原製造廠該國是否將此產品列入醫療器材管理,但為參考用,仍應以我國衛生福利部食品藥物管理署規定為主。
醫療器材依其風險程度,分級如下:
ㄧ、第一等級:低風險性。
二、第二等級:中風險性。
三、第三等級:高風險性。
【參考連結】醫療器材分類分級管理辦法
https://www.fda.gov.tw/TC/siteListContent.aspx?sid=11652&id=36489
【參考連結】非醫療器材列管之產品列表
https://www.fda.gov.tw/TC/siteListContent.aspx?sid=11652&id=36834
ENG
To determine whether a medical device is a listed medical device, you can first check the regulations on the classification and classification management of medical devices. If a product meets any of the classification items, it is listed as a medical device.
If it is an imported product, you can refer to the regulations of the importing country to determine whether the product is included in the medical device management of the original manufacturer, but for reference, it should still be based on the regulations of the Medical devices and Drug Administration of the Ministry of Health and Welfare.
Medical devices are classified as follows according to their degree of risk:
Class 1: Low risk
Class II: Medium risk
Class III: High risk
【Website】
https://www.fda.gov.tw/ENG/Site.aspx?sid=10168
HLF-TW-20
外國公司要到台灣銷售醫療器材,無論設100%子公司或分公司,需要在公司登記時取得營業特許證?
假如要,其必要條件是什麼?所需文件及申請程序為何?網頁?
If a foreign company wants to sell medical devices in Taiwan, no matter if it sets up a 100% subsidiary or branch, does it need to obtain an approval from local health bureau before the company’s registration?
If so, what are the requests?
What are the required documents and application procedures? Website?
Evershine RD:
醫療器材銷售公司要申請營業特許證,它在經濟部營業項目中的代號尾碼為1不為0。0代表不需辦理特許,1代表需要。
醫療器材批發業F108031;醫療器材零售業F208031
營業特許證依照各縣市政府規定辦理,以台北市為例:
臺北市政府衛生局「中西藥品及醫療器材販賣業籌設」所需文件:
1. 臺北市販賣業藥商、販賣業醫療器材商、藥局登錄及變更申請書1份
2. 營業場所地址及設備簡圖1份
3. 負責人國民身分證正、反面影本1份(加蓋公司大小章)
4. 公司名稱及所營事業登記預查核定書影本1份
5. 「營業場所土地使用分區管制與建築管理規定查詢表」審查核定影本1份
6. 新設立公司組織之藥商、醫療器材商附公司章程,既有之公司增加營業項目者附修正後之公司組織章程(或附股東同意書/相關會議記錄影本)1份
7. 中、西藥販賣業者應同時辦理藥師(藥劑生)執業登記,藥師(藥劑生)應親至現場
8. 藥師從事中藥製劑之製造、供應及調劑或藥劑生從事中藥之買賣及管理者,應附修習中藥課程達標準之證明文件影本1份(藥師:16學分;藥劑生:144小時)。【若證書背面左上角蓋有修習中藥學分關防或證書背面蓋有已修習中藥課程16學分戳章則免附】
9. 中醫師擔任中藥管理人(中醫師應親至現場):
(1)中醫師證書正、反面影本1份
(2)國民身分證正、反面影本1份
(3)公會會員證明文件正本1份
10. 從事輸入或維修之醫療器材販賣業者,應辦理技術人員登記:
(1)國民身分證正、反面影本1份
(2)畢業證書正、反面影本1份
(3)在職證明文件正本1份
(4)從事相關業務佐證文件1份
(5)輸入技術人員應備最近5年內至少20小時以上教育訓練佐證資料影本1份
(6)上述文件(2、4、5)至遲自113年5月1日起,應符合「醫療器材技術人員管理辦法」規定
11. 中藥販賣業者需附【遵守野生動物保育切結書】1份
12. 藥商、醫療器材商設於醫院、學校、市場等,應附該管理單位之同意書正本1份
13. 委託辦理者:委託書1份(請書明委託人及受委託人之姓名、身分證字號、地址及簽章)
【參考連結】臺北市政府衛生局「中西藥品及醫療器材販賣業籌設」
https://service.gov.taipei/Case/ApplyWay/201903210169
ENG
In the code table of the business of the Ministry of Economic Affairs, the last digit of the code is 0, which means no preliminary approval is required and 1 means that preliminary approval is required.
Medical devices wholesale F108031;Medical devices retail F208031
To apply the business license, please follow the regulations of each county and city government.
【Website】
https://service.gov.taipei/Case/ApplyWay/201903210169
HLF-TW-25
假如需要辦理,請問台灣有專業服務公司可以協助辦理醫療器材公司營業許可證?
Evershine RD:
HLF-TW-30
外國公司要到台灣銷售醫療器材,可以指派台灣公司擔任營業代理人銷售嗎? 所需文件及申請程序為何?若外國公司銷售個人自用,指無需由醫師或專業人員操作之醫療器材到台灣,所需文件及申請程序為何?醫療器材包裝內容及各種標示,需要事先核准嗎?
可允許的語文除了中文外,其他哪種語文也可以?網頁?
If a foreign company wants to sell medical devices in Taiwan, can it assign a Taiwan company to act as a sales agent? What are the required documents and application procedures?
If a foreign company sells medical devices for personal use, meaning that it does not need to be operated by a doctor or professional, to Taiwan, what are the required documents and application procedures?
Do the packaging contents and various labels of medical devices require prior approval?
In addition to Chinese, which other languages are allowed? Web page?
Evershine RD:
依據「醫療器材管理法施行細則」第8條中規定,申請醫療器材查驗登記須以醫療器材商名義提出申請。
申請專供個人自用,指無需由醫師或專業人員操作,由自然人自行使用之醫療器材。申請之數量,應符合下列規定:
(一)專供個人自用之耗材類,其數量以6個月用量為限。
(二)專供個人自用之儀器類,同一型號以一部(個)為限。但有於不同地點使用或有其他特殊情形必要者,不在此限。
申請專供個人自用之醫療器材者,其應檢附之文件、資料如下:
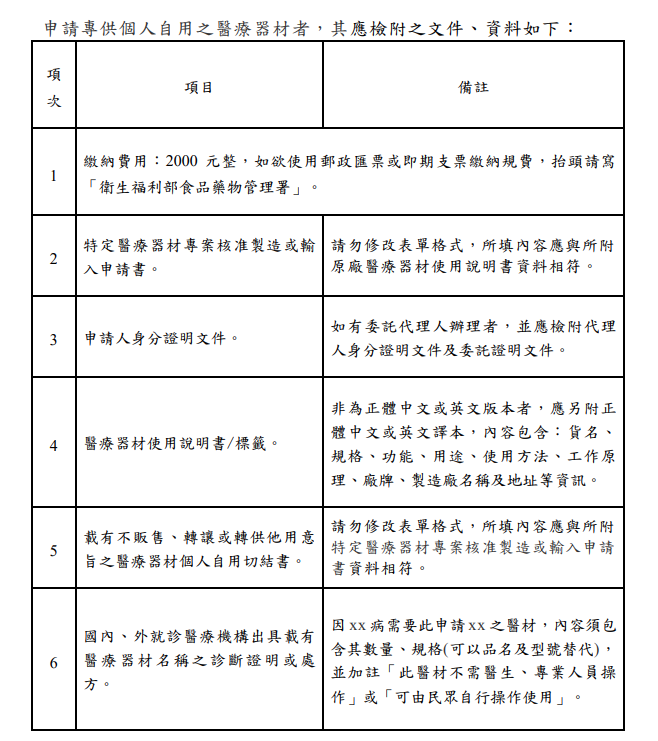
申請專供個人自用醫療器材專案製造或輸入符合下列規定之一者,得以中央主管機關公告之便捷通關管理方式輸入:
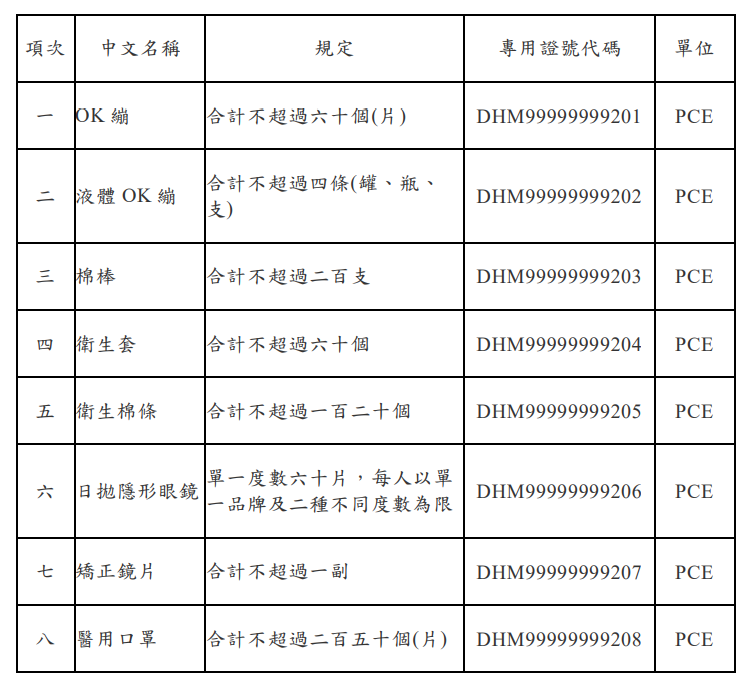
【參考連結】醫療器材管理法
https://www.fda.gov.tw/TC/siteList.aspx?sid=11652&scid=791
【參考連結】醫療器材管理法施行細則
https://www.fda.gov.tw/TC/siteList.aspx?sid=11652&scid=791
【參考連結】特定醫療器材專案核准製造及輸入辦法
https://www.fda.gov.tw/TC/siteListContent.aspx?sid=11652&id=36475
ENG
According to Article 8 of the “Enforcement Regulations of the Medical Device Management Act”, an application for medical devices inspection and registration must be filed in the name of a medical device manufacturer
The application is exclusively for personal use, which refers to medical devices that does not need to be operated by a doctor or professional. The number of applications shall meet the following requirements:
(1) Consumables exclusively for personal use, the quantity of which is limited to 6-month consumption.
(2) Instruments designed for personal use, limited to one (piece) of the same model. However, it is not limited to use in different locations or other special circumstances.
Applicants who apply for medical devices exclusively for personal use shall submit the following documents and materials:
Those applying for the project manufacture or import of medical devices for personal use that meet one of the following requirements can be imported through the convenient customs clearance management method announced by the central competent authority:
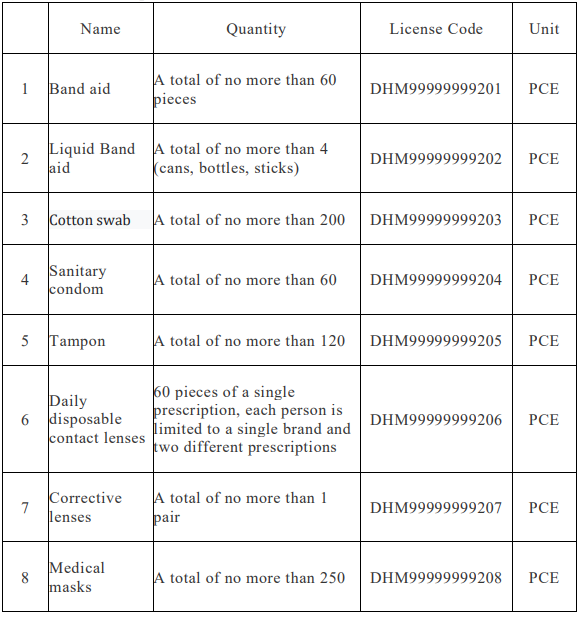
【Website】
https://www.fda.gov.tw/ENG/Site.aspx?sid=10168
HLF-TW-35
假如需要辦理指派台灣公司擔任營業代理人,請問台灣有專業服務公司可以協助?
Evershine RD:
HLF-TW-40
不同等級之醫療器材所需文件及請程序為何?網頁?
What are the required documents and application procedures for different classes of medical devices? Web page?
Evershine RD:
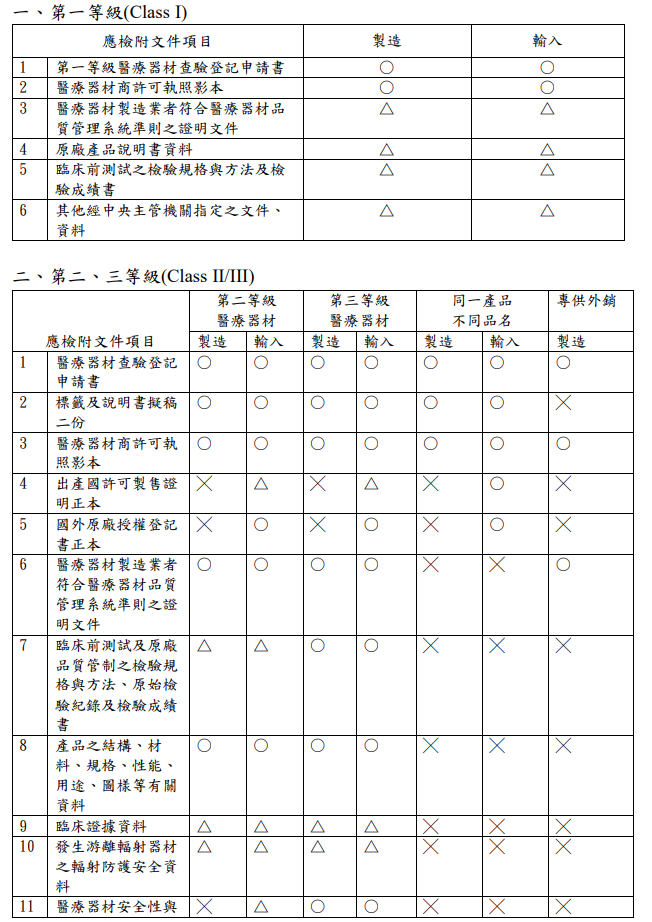
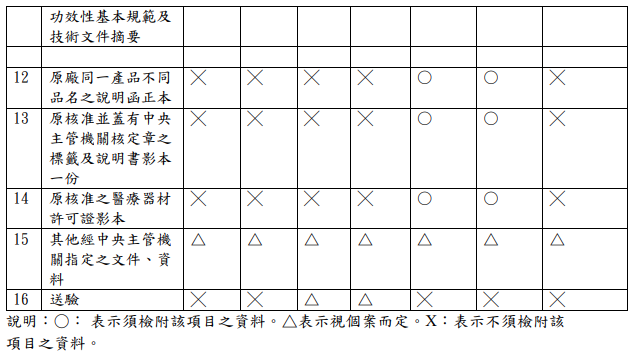
【參考連結】申請查驗登記表單
https://www.fda.gov.tw/TC/site.aspx?sid=11682&r=904891393
ENG
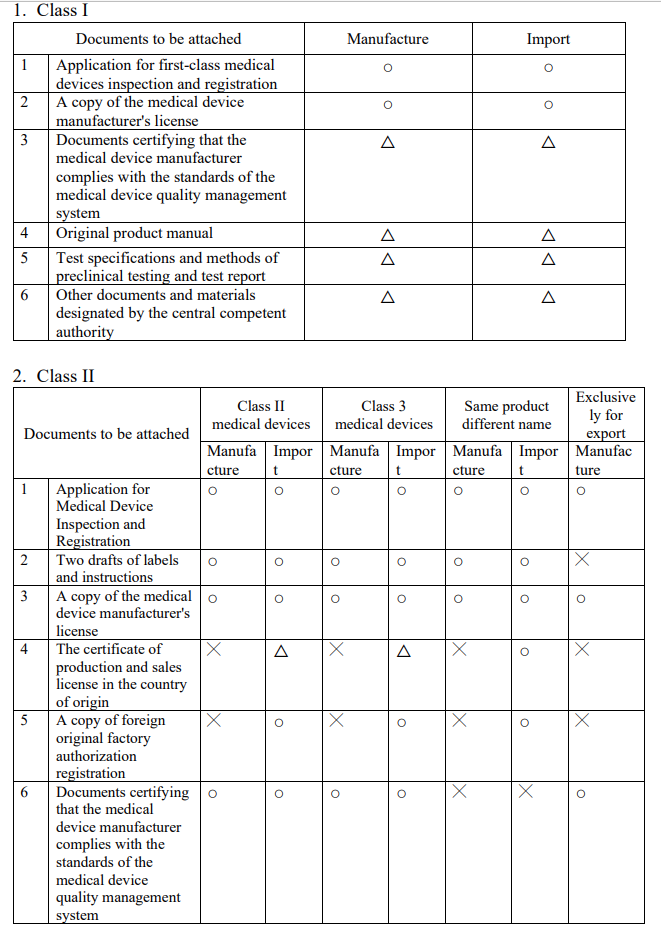
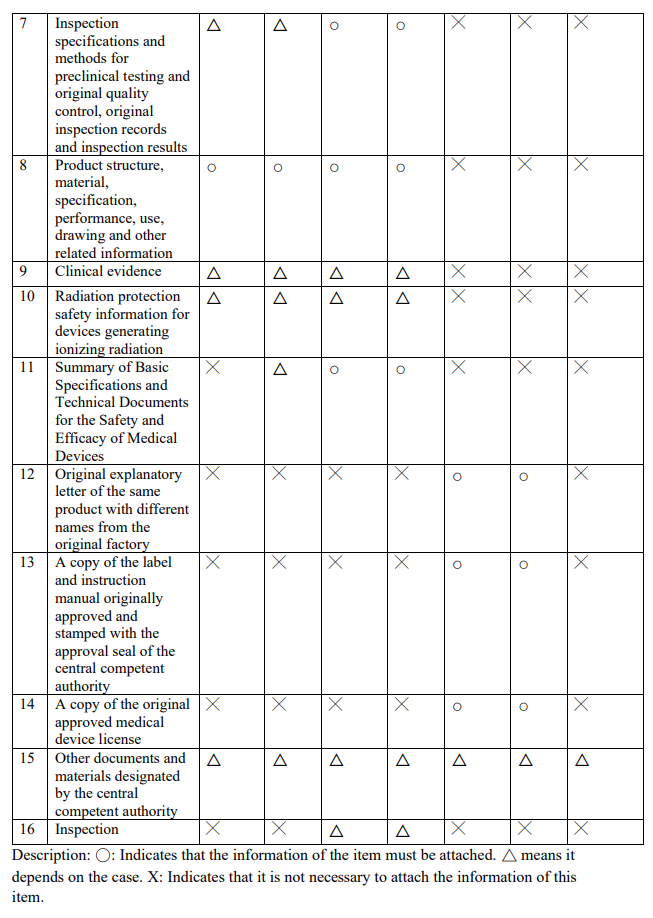
【Website】
https://www.fda.gov.tw/ENG/Site.aspx?sid=10168
HLF-TW-45
請問在台灣有哪些專業服務機構,可以協助辦理醫療器材產品許可證?
Evershine RD:
HLF-TW-50
醫療器材可否在網路平台販售,其相關規定為何? 網頁?
Can medical devices be sold on online? What are the relevant regulations? Website?
本規定依醫療器材管理法第十八條規定訂定之。
一、通訊交易通路:指透過廣播、電視、電話、傳真、型錄、報紙、雜誌、網際網路、傳單或其他類似之方法,使消費者未能實際檢視醫療器材而為買賣之通路;通訊交易通路業者:指提供通訊交易通路予醫療器材商(藥局)從事醫療器材販售業務之業者。
二、欲於通訊交易通路販售醫療器材者,應符合下列資格之一:
(1) 核准登記之醫療器材商。
(2) 核准登記之藥局。
三、於前點通路販售之醫療器材,以第一等級及附件所列之第二等級醫療器材品項為限。
四、 醫療器材商(藥局)於通訊交易通路販售醫療器材,應同時於其通路提供消費者下列資訊:
(1) 醫療器材品名、許可證字號或登錄字號、許可證所有人或登錄者之名稱及地址、製造業者名稱及地址。(2) 醫療器材商(藥局)之名稱、地址、許可執照字號及諮詢專線電話。
(3) 加註「消費者使用前應詳閱醫療器材說明書」。
(4) 具量測功能之產品,其定期校正服務之項目及據點資訊。
醫療器材商及藥局得於通訊交易通路販售之第二等級醫療器材品項
| 項次 | 品項代碼* | 名稱 | 產品示例 |
| 1 | E.2770 | 阻抗式體積描記器(阻抗式週邊血流描記器) | 體脂計 |
| 2 | L.5300 | 衛生套(保險套) | 保險套 |
| 3 | L.5310 | 含殺精劑的衛生套 | 保險套 |
| 4 | L.5460 | 具香味或除臭的衛生棉塞 | 衛生棉條 |
| 5 | L.5470 | 無香味的衛生棉塞 | 衛生棉條 |
| 6 | I.4040 | 醫療用衣物 | 手術用口罩、手術用N95口罩 |
| 7 | I.0004 | 酒精棉片 | 酒精棉片、酒精棉球 |
| 8 | I.0005 | 優碘棉片 | 優碘棉片、碘液棉棒、碘液紗布 |
| 9 | I.4014 | 外部使用非吸收式紗布或海綿球 | 凡士林紗布 |
| 10 | J.5240 | 醫療用黏性膠帶及黏性繃帶 | 免縫膠帶 |
| 11 | M.5918 | 硬式透氣隱形眼鏡保存用產品 | 硬式隱形眼鏡清潔液、保養液、保存液、護理液、濕潤液、雙氧系統、去蛋白錠、隱形眼鏡用緩衝生理食鹽水 |
| 12 | M.5928 | 軟式隱形眼鏡保存用產品 | 軟式隱形眼鏡清潔液、保養液、保存液、護理液、濕潤液、雙氧系統、去蛋白錠、隱形眼鏡用緩衝生理食鹽水 |
| 13 | 醫療器材軟體 | 第二等級醫療器材軟體 | |
| 14 | E.1120 | 血壓壓脈帶 | 血壓壓脈帶、血壓袖帶、血壓量測臂帶 |
| 15 | L.5400 | 月經量杯 | 月經杯、月事杯、月亮杯 |
| 16 | O.3800 | 醫療用電動代步器 | 醫療用電動代步車 |
| 17 | O.3860 | 動力式輪椅 | 電動輪椅、安裝於輪椅之電動輔助推行器 |
| 18 | G.5220 | 耳鼻喉佈施藥裝置及其搭配使用之物質 | 海水洗鼻器、海水鼻用噴霧器、洗鼻鹽 |
| 19 | J.2910 | 臨床電子體溫計 | 耳溫槍、耳溫槍專用耳套、額溫槍 |
【參考連結】醫療器材管理法
https://www.fda.gov.tw/TC/siteList.aspx?sid=11652&scid=791
【參考連結】通訊交易通路販售醫療器材之品項及應遵行事項
https://www.fda.gov.tw/TC/siteContent.aspx?sid=11680
ENG
These regulations are formulated in accordance with Article 18 of the Medical Devices Act.
1. Communication transaction channel: refers to the channel through which consumers can buy and sell medical devices without actually viewing medical devices through radio, television, telephone, fax, catalogs, newspapers, magazines, the Internet, leaflets or other similar methods; communication Trading channel operators: refer to the operators that provide communication trading channels to medical device manufacturers (pharmacies) engaged in the sales of medical devices.
2. Those who want to sell medical devices through communication channels should meet one of the following qualifications:
(1) Approved registered medical device suppliers.
(2) Approved and registered pharmacies.
3. The medical devices sold in the front channel is limited to the first-class medical devices and the second-class medical devices listed in the appendix.
4. Medical device dealers (pharmacies) that sell medical devices through communication channels should also provide consumers with the following information on their channels:
(1) The name of the medical device, the license number or registration number, the name and address of the license owner or registration person, and the name and address of the manufacturer.
(2) The name, address, license number and consultation hotline of the medical device manufacturer (pharmacy).
(3) Add “Consumers should read the instruction manual of the medical device carefully before using it”.
(4) For products with measurement function, the items and base information of the service are regularly corrected.
Class II medical devices items that medical devices manufacturers and pharmacies can sell through communication channels
| Item | Code* | Name | Example |
| 1 | E.2770 | Impedance Plethysmograph (Impedance Peripheral Blood Flow Logger) | Body fat meter |
| 2 | L.5300 | Sanitary condoms (condoms) | Condom |
| 3 | L.5310 | Sanitary condoms with spermicide | Condom |
| 4 | L.5460 | Scented or deodorized tampons | Tampon |
| 5 | L.5470 | Fragrance-free tampons | Tampon |
| 6 | I.4040 | Medical clothing | Surgical masks, Surgical N95 masks |
| 7 | I.0004 | Alcohol pads | Alcohol cotton pads, Alcohol cotton balls |
| 8 | I.0005 | Betadine cotton sheet | Betadine cotton sheet, Iodine liquid cotton stick, Iodine liquid gauze |
| 9 | I.4014 | External use of non-absorbent gauze or sponge | Vaseline gauze |
| 10 | J.5240 | Medical adhesive tapes and adhesive bandages | Seam-free tape |
| 11 | M.5918 | Products for storage of rigid breathable contact lenses | Hard contact lens cleaning solution, maintenance solution, preservation solution, nursing solution, moisturizing solution, hydrogen peroxide system, deproteinized tablet, buffered saline solution for contact lenses |
| 12 | M.5928 | Soft contact lens storage products | Soft contact lens cleaning solution, maintenance solution, preservation solution, nursing solution, moisturizing solution, hydrogen peroxide system, deproteinized tablet, buffered saline solution for contact lenses |
| 13 | Medical devices software | Class II medical device software | |
| 14 | E.1120 | Blood pressure cuff | Blood pressure cuff, blood pressure cuff, blood pressure measurement armband |
| 15 | L.5400 | Menstrual cup | Menstrual cup, Menstrual cup, Moon cup |
| 16 | O.3800 | Medical electric scooter | Medical electric scooter |
| 17 | O.3860 | Power Wheelchair | Electric wheelchair, electric assisted pusher mounted on wheelchair |
| 18 | G.5220 | Ear, nose and throat drug delivery devices and substances used in conjunction therewith | Sea water nasal wash, sea water nasal spray, nasal wash salt |
| 19 | J.2910 | Clinical electronic thermometer | Ear thermometer, earmuffs for ear thermometer, forehead thermometer |
【Website】https://www.fda.gov.tw/ENG/Site.aspx?sid=10168
各國醫療器材登記法規問答集
聯絡我們:
台北永輝協同網路服務股份有限公司永輝啟佳聯合會計師事務所地址:104臺北市中山區長春路378號6樓
捷運文湖線南京復興站,兄弟大飯店附近
聯絡人: 朱鍵彰 協理
電話 : +886-2-2717-0515 E103
手機 : +886-939-357-735
電郵:sales.taiwan@evershinecpa.com
全球永輝服務據點參考資料:
永輝100%關係企業
永輝總部、臺北永輝、廈門永輝、北京永輝、上海那靈、深圳常新、紐約永輝、加州永輝、德州永輝、鳳凰城永輝、東京永輝、首爾永輝、河內永輝、越南胡志明、曼谷永輝、新加坡永輝、吉隆玻永輝、雅加達永輝、馬尼拉永輝、墨爾本永輝、澳洲雪梨、孟加拉永輝、新德里永輝、印度孟買、杜拜永輝、法蘭克福永輝、巴黎永輝、倫敦永輝、荷蘭永輝、西班牙永輝、義大利永輝、羅馬尼亞永輝、多倫多永輝、墨西哥永輝、墨西哥永輝。
其他已提供中文化服務城市:
邁阿密、亞特蘭大、俄克拉荷馬、密歇根、西雅圖、特拉華; 柏林; 斯圖加特;布拉格;布加勒斯特;班加羅爾;泗水; 高雄、香港、深圳、東關、廣州、清遠、永康、杭州、蘇州、崑山、南京、重慶、許昌、青島、天津。
永輝潛在可服務城市 (2個月籌備期):
我們為IAPA會員所,總部在倫敦,全球300個會員所,員工約1萬人。
我們為LEA會員所,總部在美國芝加哥,全球600個會員所,員工約2萬8千人。
Evershine is local Partner of ADP Streamline® in Taiwan. (版本:2022/03)
請用下列電郵與我們聯繫: HQ4TPE@evershinecpa.com